Two-Way Messaging Available! Text Us At: (602) 840-0111
Download our NEW Mobile App!
Get Healthy!
- I. Edwards
- Posted April 17, 2025
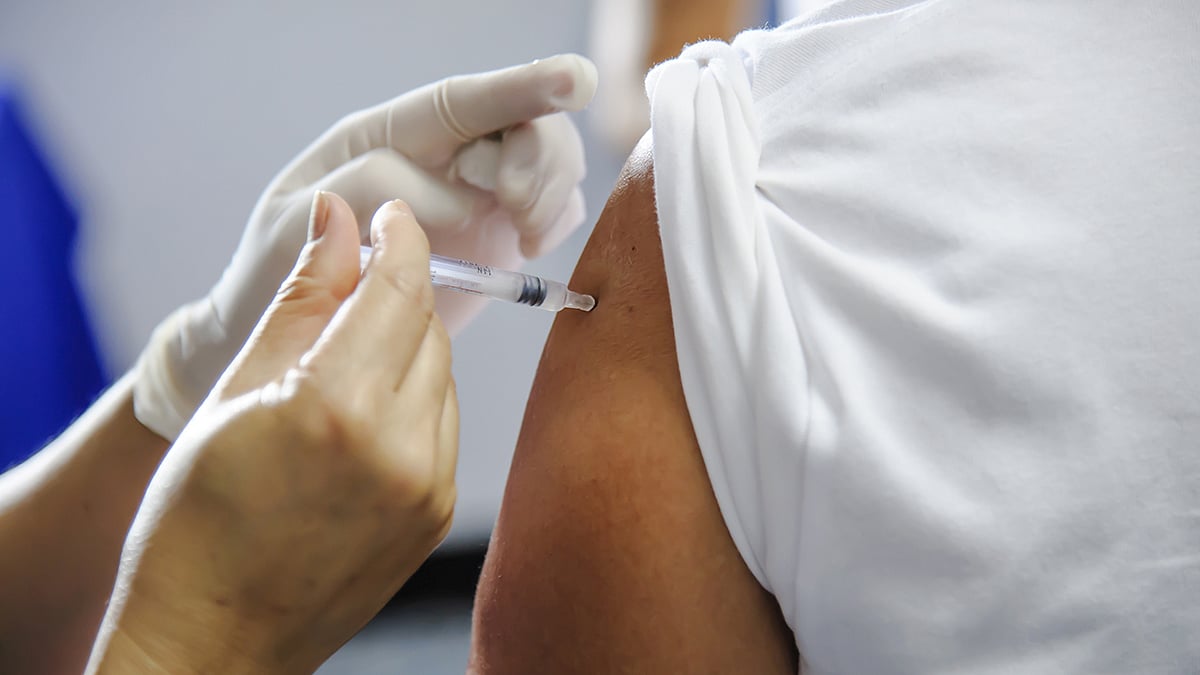
Vaccine Panel Urges More Protection Against RSV, Meningitis and Chikungunya
A panel of federal health experts on Wednesday recommended expanded vaccine options for several diseases, including respiratory syncytial virus (RSV), meningitis and a mosquito-borne illness called chikungunya.
The panel, known as the Advisory Committee on Immunization Practices (ACIP), makes vaccine use recommendations to the director of the U.S. Centers for Disease Control and Prevention (CDC), The Associated Press reported.
These recommendations are almost always approved. But recent leadership changes at the CDC may complicate next steps.
Acting Director Susan Monarez, who is President Donald Trump's pick to lead the CDC, awaits U.S. Senate confirmation. Until that happens, she has recused herself from regular duties due to federal law regarding vaccines.
According to two CDC officials who spoke anonymously, decisions may now fall to U.S. Health Secretary Robert F. Kennedy Jr., a longtime anti-vaccine activist.
The new recommendations include:
Adults aged 50 to 59 with certain health problems, like heart disease, diabetes or chronic obstructive pulmonary disorder should be able to get the RSV shot.
A new combination vaccine from GSK was endorsed. It protects against five types of meningococcal bacteria, including one linked to college campus outbreaks about 10 years ago.
A second vaccine for chikungunya was recommended for travelers age 12 and older heading to countries with current outbreaks. About 100 to 200 cases of chikungunya are reported each year among U.S. travelers.
A safety warning was added to an older chikungunya vaccine that uses a weakened live virus. People 65 and older should discuss the risks with their doctor, especially if they have other health conditions. This follows reports of six older adults developing heart or brain symptoms shortly after getting the shot. The cases are stil being investigated.
More information
Learn more about vaccines at the Mayo Clinic.
SOURCE: The Associated Press, April 16, 2025





